Advancements in Cell Culture: Choosing Between Adherent and Suspension Platforms
Currently, there are two main cell culture systems: one allows cells to grow in a single layer on artificial substrates (adherent culture), and the other allows cells to grow freely suspended in the culture medium (suspension culture). Each of these methods is suitable for different research applications, such as basic cell biology, disease modeling, and biopharmaceutical production (including bioproduct manufacturing, cell and gene therapy, and vaccine production). Each method has its own advantages and limitations to consider, such as scalability, resource requirements, facility space, and process monitoring. This article will provide an overview of these factors.
Adherent Cell Culture
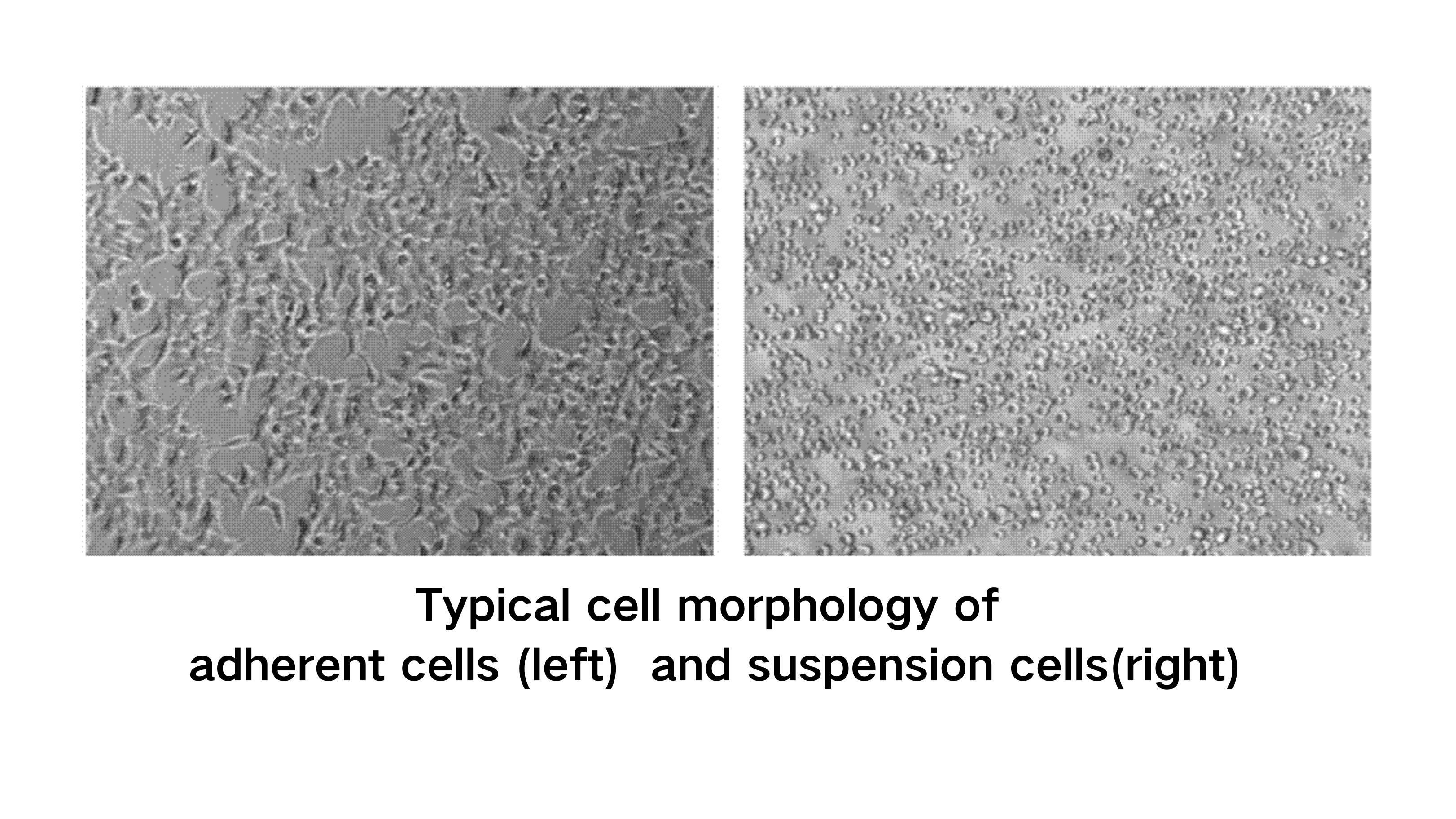
Adherent cell culture is a method for propagating cell types that rely on attachment to a growth substrate. Cells that depend on attachment to the substrate or extracellular matrix (ECM) for proliferation and survival are known as adherent cell types. Many common primary and continuous cell lines are adherent, including mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), human embryonic kidney 293 (HEK293) cells, Vero cells, and Chinese hamster ovary (CHO) cells. Adherent cell types can be categorized into several groups based on their origin and morphological characteristics, such as cell nucleus size, cytoplasmic ratio, and shape. For example, fibroblast-like cells are elongated and spindle-shaped, epithelial cells are polygonal, endothelial cells are arranged in a tube-like pattern, and neuronal cells extend and form projections. Many adherent platforms allow monitoring of cell morphology, which is valuable because cell morphology is an important phenotypic indicator of cell biology and function.
Intracellular signaling cascades that regulate many cellular functions, including cell cycle progression, cell differentiation, and response to growth factors, are regulated by cell-cell and cell-matrix interactions. Adherent cells provide a more natural environment for cell culture because they can recapitulate these interactions. Container surfaces can be modified with various treatments and related biological coatings to optimize cell attachment for specific cell types and experimental conditions. In this way, adherent cell culture containers can be customized to achieve or maintain specific cell phenotypes, especially for iPSCs, which often exhibit morphological changes when cultured on different surfaces. Gelatin-coated surfaces can promote endothelial cell differentiation, while hydrogels based on natural ECM can provide signals to maintain pluripotency.
Adherent culture is crucial for applications that require strict control over cell physiology and function. In regenerative medicine and tissue engineering applications, structural scaffolds are needed to support cell growth and differentiation. For example, expanding MSCs on ECM hydrogels for bone and cartilage repair, or culturing induced pluripotent stem cells (iPSCs) on adherent substrates to generate cardiomyocytes for cardiac tissue repair. Additionally, fibroblast and keratinocyte cultures on collagen matrices can produce skin grafts for wound healing, highlighting the importance of adherent culture in maintaining tissue structure.
Adherent platforms offer flexibility not only in surface modifications but also in size range to accommodate various experimental needs. Traditional culture dishes, flasks, and multiwell plates are widely used for various research applications and drug screening. For large-scale production, roller bottles remain one of the main platforms for vaccine production. Stackable platforms (e.g., CellSTACK culture chambers, Corning) and more advanced technologies have expanded traditional flat adherent culture by stacking multiple layers within the same footprint. Fixed-bed bioreactors combine adherent matrices with process automation and flow control in bioreactor vessels to achieve higher yields and larger scales. In summary, adherent platforms offer a variety of options at different scales, from research and development to clinical production, to accommodate different budgets and space requirements.

However, adherent platforms still have limitations in scalability. As cells adhere to the growth substrate, expansion of the culture is limited by surface area. When cells cover the growth surface, subculturing is necessary to disrupt cell-cell and cell-matrix connections, typically achieved through chemical methods (e.g., EDTA), enzymatic methods (e.g., trypsin), or mechanical dissociation (e.g., scraping). Many cell types tolerate subculture well. However, care must be taken to minimize degradation of surface proteins, as this can reduce the ability of cells to attach to subsequent culture vessels and adversely affect downstream applications such as surface marker identification. Subculturing adherent cells also requires additional processing steps, such as washing, incubation, stopping reactions, and/or centrifugation to remove dissociation reagents.
Labor is also a limiting factor in expanding adherent platforms. For certain container types (e.g., multi-layered containers), expansion of the culture surface area is achieved by adding multiple units of the same container rather than gradually increasing the container size. Therefore, compared to suspension platforms, expanding adherent cultures requires more manual labor and occupies larger footprint per unit growth area.
Additionally, there are some additional limitations to consider: use of culture media and open/closed systems. Some adherent culture vessels may have fixed or stricter media volume requirements. However, combining circulation and process monitoring can help adjust media usage to levels more favorable for specific adherent platform growth areas. Finally, many traditional adherent containers are handled as open systems. While handling culture dishes and bottles
in small-scale open systems may not be an issue, manual handling of roller bottles and larger stackable containers poses contamination risks. For large-scale expansion processes, closed system configurations and accessories are increasingly applicable to some adherent containers to mitigate contamination risks during large-scale expansion processes.
Suspension Cell Culture
Suspension culture is a method in which cells are suspended in growth medium (Figure 1). Suspension cells do not need to adhere to a substrate; they grow freely either as individual cells or as aggregates or clusters of multiple cells. This mode of growth is the natural state of hematopoietic stem cells and immune cells (such as Jurkat cells). Various insect cell lines, such as Sf9 cells, can also grow in suspension. CHO and similar production cell lines have been adapted to grow in suspension. However, not all adherent cell lines are suitable for suspension culture domestication. For example, epithelial cells lose polarity and the ability to form connections in suspension culture, affecting their function.
Generally, suspension cells require some form of agitation during culture to prevent settling to the bottom of the container and to promote gas and nutrient exchange. They can be cultured in several different dynamic culture platforms. One is shaking flasks, such as Erlenmeyer flasks with gas-permeable caps, which rotate the culture medium when placed on an orbital shaker. Spinner flasks and bioreactors use agitators to mix the culture, keeping it aerated and in suspension. Suspension culture can also be performed in culture bags on rocking bioreactor platforms. Depending on scale and container type, gas exchange can occur passively through diffusion in the atmospheric air of the incubator or actively through providing controlled gas mixtures.
Because suspension culture is dynamic, it imposes greater shear stress on cultures compared to static adherent culture, and some cell types, particularly primary cells and stem cells, are more sensitive than others. Therefore, surfactants such as polyvinyl alcohol are often added to suspension culture media to protect cells from shear stress. Similarly, different suspension platforms generate varying degrees of shear stress. Design variations can reduce shear stress, such as shaking flasks with or without baffles, different agitator designs, and traditional stirred bioreactors versus vertical-wheel bioreactors (e.g., Vertical-Wheel, PBS Biotech). Gas sparging methods and rates also affect shear stress in actively aerated cultures. Suspension culture requires a balance between providing sufficient agitation to aerate the culture medium and prevent settling while minimizing shear stress on the cells.
In addition to shear stress limiting cell expansion, culture volume and cell density can also limit suspension culture. For this, suspension cells are simply diluted with fresh media to perform cell passaging, maintaining a specific density of live cells per unit volume to sustain growth and keep cells in the logarithmic growth phase. Particularly in production-scale stirred reactors, suspension cell culture offers significantly higher yields and space efficiency compared to adherent culture. These platforms operate as closed systems with process monitoring and control capabilities. Process monitoring can accurately track cell expansion progress without direct cell observation by conducting sufficient process development to understand and characterize the growth characteristics of each suspension cell line.
The scalability and operational efficiency advantages of suspension platforms come with upfront time and labor costs. On one hand, suspension culture plays a critical role in biopharmaceutical production due to its scalability. CHO cells in suspension culture are widely used for monoclonal antibody (mAb) production, treating diseases from cancer to autoimmune diseases. Additionally, suspension culture of insect cells facilitates rapid vaccine production, such as recombinant protein vaccines for human papillomavirus. However, other cell types requiring adaptation to suspension culture still require time and resources for cell line and application development. Although advances in technology have blurred the boundaries between adherent and suspension cell culture, there has been a shift towards more scalable suspension culture in some traditional adherent applications. For example, adenovirus vectors for cell and gene therapy applications, once reliant on adherent HEK293 culture, are now transitioning to using suspension HEK293 cell lines and commercial suspension transfection reagents for higher yields.
Advanced platforms offer a hybrid approach
Microcarrier technology is a hybrid method that enables adherent cells to be cultured in scalable suspension environments. Microcarriers are small particles used to provide a growth substrate for adherent cells to grow in suspension. Microcarriers can be made from various materials, including polystyrene and dextran-based microcarriers. Other microcarriers are made from biodegradable polymers, simplifying downstream processing with no need for separation steps. Surface modifications of microcarriers range from simple protein coatings (e.g., collagen or fibronectin) to more complex synthetic peptides mimicking components of the ECM, each design aimed at optimizing the growth and function of specific cell types. Importantly, microcarriers enable traditionally adherent cells to be cultured in suspension for monoclonal antibody production.
.jpg)
Another hybrid method between adherent and suspension culture is the cultivation of spheroids and organoids, also known as 3D cell culture. There are some methods that utilize ECM scaffolds to support 3D growth, while others are scaffold-free methods that promote cell organization mimicking the morphology and function of in vivo tissues. For example, human induced pluripotent stem cells (hiPSCs) cultured as spheroids in suspension can differentiate into hiPSC-derived cardiomyocytes. Regardless of the method used, cell-cell connections are formed in three-dimensional space, providing the support needed for more natural cell behavior and improving gradients of oxygen, nutrients, and signaling molecules crucial for cell differentiation and function. The tissue-like structures formed in 3D culture provide advanced models with high physiological relevance for studying disease mechanisms, tissue regeneration, and drug responses compared to 2D systems.
Conclusion
The development of cell culture technologies continues to innovate biological research and industrial applications. While adherent platforms that embody the advantages of natural cell-cell and cell-matrix interactions have limitations in scalability and high labor demands, suspension culture excels in scalability, especially for high-yield production, provided that cell types can grow in suspension. Advanced platforms that integrate the advantages of both are leading to more advanced and efficient culture systems. Choosing the right platform requires a deep understanding of their respective advantages and limitations to ensure alignment with the intended applications and research goals. Looking ahead, ongoing innovation is expected to further enhance these cell culture technologies, expanding their applications in biopharmaceuticals, regenerative medicine, and cell and gene therapy fields. This progress also underscores the critical role of strategic platform selection in realizing the full potential of cell culture advancements.

















