Understanding Colloidal Gold: Preparation, Characteristics, and Application Exploration
Colloidal gold is composed of colloidal gold nanoparticles, formed when gold particles are dispersed in an aqueous solution, a state also known as colloidal gold. Chlorauric acid is used to produce colloidal gold under the influence of reducing agents (such as white phosphorus, ascorbic acid, sodium citrate, tannic acid, etc.), reducing Au3+ to gold atoms. Once the solution reaches saturation, nanoscale gold particles, or gold nuclei, precipitate, with the remaining gold atoms adhering to these nuclei and polymerizing into specific-sized gold particles, achieving stability through electrostatic forces.

The core of colloidal gold is not perfectly spherical; smaller colloidal gold particles are typically spherical, while larger particles (usually greater than 30nm) tend to be elliptical. The size of the gold particles produced can be controlled using different types and concentrations of reducing agents. Colloidal gold exhibits three unique nanomaterial effects: surface effect, small size effect, and macroscopic quantum tunneling effect, offering a large surface area and possessing unique optical, electrical, thermal, and other physical properties, along with good biocompatibility.
Due to its color rendering and high specificity and sensitivity in binding reactions with antigens, colloidal gold-based solid-phase carrier immunochromatographic rapid detection technology has been developed.
Production of Colloidal Gold
Colloidal gold is easy to prepare, cost-effective, and undergoes rapid visual color formation. It remains stable in both liquid and dry states and is resistant to fading. Currently, colloidal gold production methods are broadly categorized into dispersion and reduction methods. The reduction method is widely employed, utilizing reducing agents to convert gold ions into gold atoms. Commonly used reduction methods include white phosphorus reduction, ascorbic acid reduction, trisodium citrate reduction, tannate-trisodium citrate reduction, ethanol ultrasonic reduction, and sodium borohydride reduction.
For instance, 16nm colloidal gold prepared via the trisodium citrate reduction method involves heating 100ml of 0.01% chloroauric acid aqueous solution to boiling and adding 2ml of 1% trisodium citrate aqueous solution under magnetic agitation. This process leads to the formation of nanoscale gold particles. The size of the colloidal gold particles depends on the amount of sodium citrate added during preparation, with different doses yielding particles of varying sizes.
The size of colloidal gold particles is also influenced by the ratio of chlorauric acid to trisodium citrate solution during preparation. By maintaining a fixed chlorauric acid dosage and adjusting the trisodium citrate dosage, particle size can be controlled.
Different sizes of colloidal gold particles serve different purposes: particles ranging from 3-15nm are suitable for electron microscope-level marking detection, those larger than 15nm for cell-level marking detection, and those exceeding 20nm for naked-eye-level marking detection.
Precautions for Colloidal Gold Production
Chlorauric acid is hygroscopic and should be stored away from light.
Use high-quality deionized or ultra-pure water for colloidal gold preparation.
Filter the solution using a 0.45μm filter membrane to remove polymers and impurities.
Prepare and use reducing agents appropriately.
Chlorauric acid is corrosive to metals; therefore, glassware should be used during preparation and storage.
Ensure the absolute cleanliness of equipment during preparation and storage.
Use a constant temperature heater to maintain uniform temperature.
Control stirring speed to ensure uniform concentration and temperature, avoiding vortex formation and splashing.
Colloidal Gold Labeling
Colloidal gold labeling involves attaching gold particles to the surface of macromolecules to create electron density markers for easy observation. Three forces primarily drive colloidal gold binding: electrostatic action, hydrophobic action, and gold-sulfur bonding. The binding of colloidal gold to proteins does not affect protein biological activity.
During labeling, the pH of ligands and colloidal gold is critical. The pH of ligand and colloidal gold solutions should be slightly higher than the ligand's isoelectric point. A pH below the isoelectric point causes agglutination, while a pH above it limits colloidal gold-protein ligand adsorption due to charge repulsion. Therefore, reactions with ligands should be conducted using colloidal gold solutions with varying pH values. Agglutination is observed when the reaction changes from red to gray-purple, with the minimum pH value before agglutination being optimal.
The colloidal gold-to-protein ligand ratio also affects labeling. The optimal protein amount is determined visually. After sufficient protein coating on gold particle surfaces, sodium chloride addition should not destroy the particles, and the color remains red. By adding a series of diluted protein solutions under optimal pH, sodium chloride is introduced, and visible precipitation indicates the minimum protein concentration before precipitation, which is the optimal protein concentration for colloidal gold. The ligand amount is then increased by 20%.
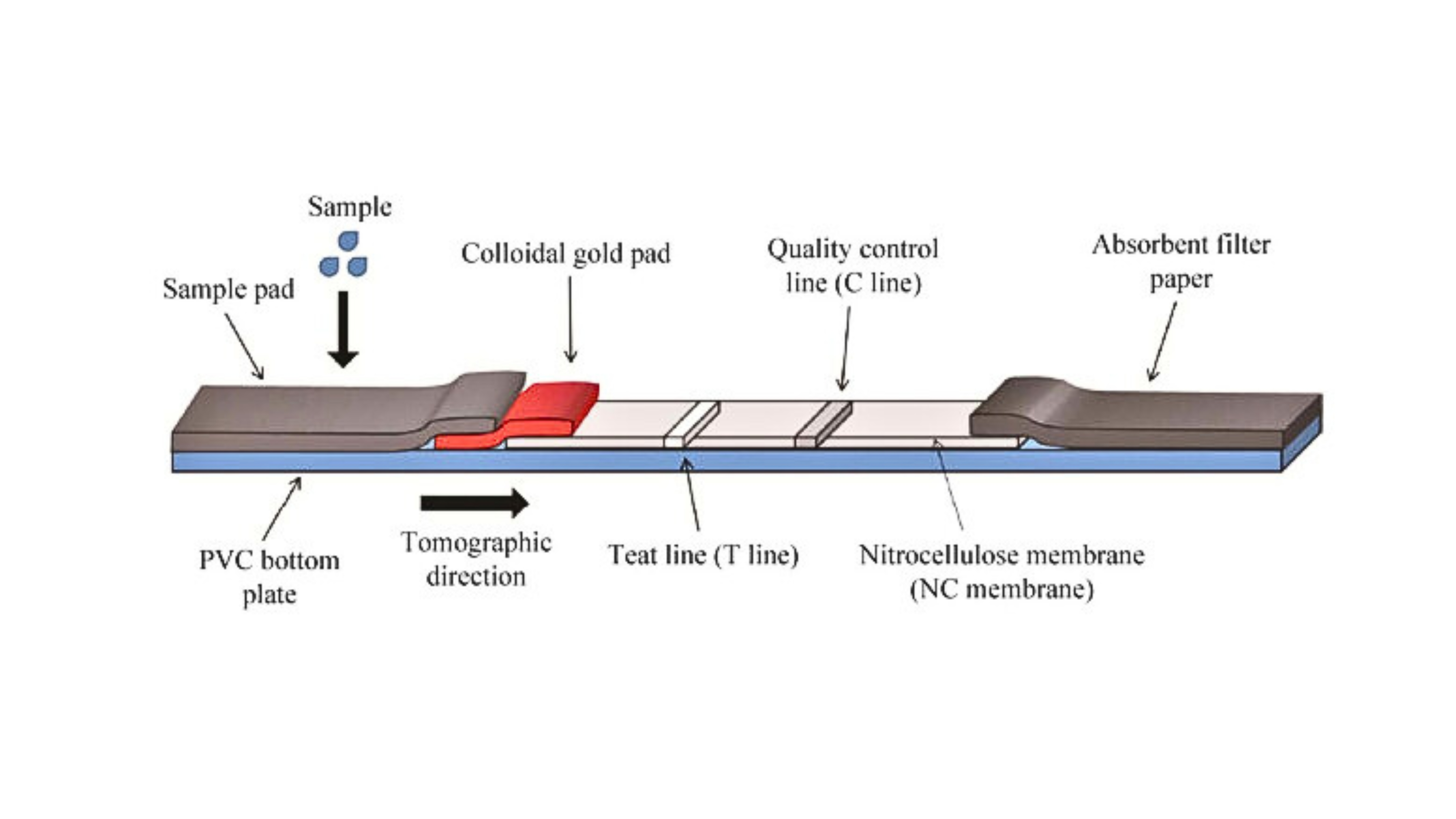
Colloidal Gold Product Introduction
Influenza is an acute respiratory infection caused by the influenza virus, mainly spread through droplets in the air, person-to-person contact, or contact with contaminated objects. The colloidal gold immunochromatography double antibody sandwich technology is employed in a product designed for qualitative detection of influenza A and B virus antigens in human throat swab samples. Results can be obtained within 5-10 minutes of sample addition.
The fecal occult blood (FOB) test kit is used to evaluate gastrointestinal bleeding clinically. It requires no supporting instruments, offers rapid detection, simple operation, and flexible application across multiple scenarios. It enables effective and timely monitoring of diseases and intervention measures to reduce disease progression and mortality rates.


















